Genicular Artery Embolization Using Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Prospective Study
Purpose
To assess the feasibility and safety of genicular artery embolization (GAE) using mesenchymal stem cells in patients with bilateral knee osteoarthritis (OA).
Materials and Methods
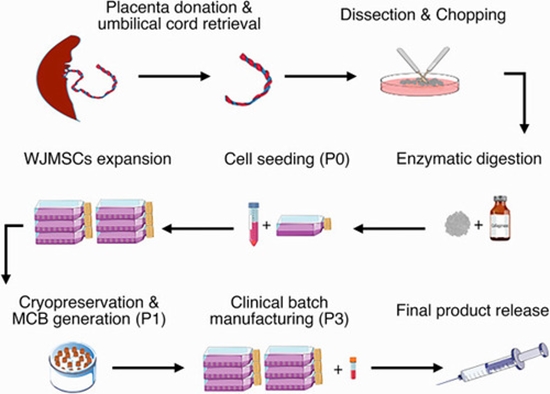
Thirty patients diagnosed with Kellgren-Lawrence Stage 3 bilateral knee OA were enrolled. GAE was performed unilaterally in each subject using 70 million cells per injection (26 descending genicular arteries, 2 inferior medial genicular arteries, and 2 superior medial genicular arteries). Technical success was defined by successful delivery of cells. Safety was evaluated by documentation of procedure-related or subsequent adverse events. Clinical outcomes were assessed with the visual analog scale and Western Ontario and McMaster Universities Osteoarthritis Index before treatment and during the subsequent 12 months. Magnetic resonance (MR) imaging was performed preprocedurally and 3 months postprocedurally. The untreated knee served as an internal control for each subject.
Results
Technical success was achieved in 100% of cases. There were two Society of Interventional Radiology (SIR) Grade 1 procedure-related adverse events (temporary skin discoloration). There were no adverse events during the study follow-up period. Significant improvements were observed in Western Ontario and McMaster Universities Osteoarthritis Index scores (29.6 [SD ± 11.5] to 4.6 [SD ± 4.5] at 12 months; P < .001) and visual analog scale scores (6.8 [SD ± 1.6] to 2.0 [SD ± 1.5] at 12 months; P < .001).
Conclusions
GAE was safely performed using mesenchymal stem cells. The mechanism of action and durability of response remain uncertain




ارسال نظر